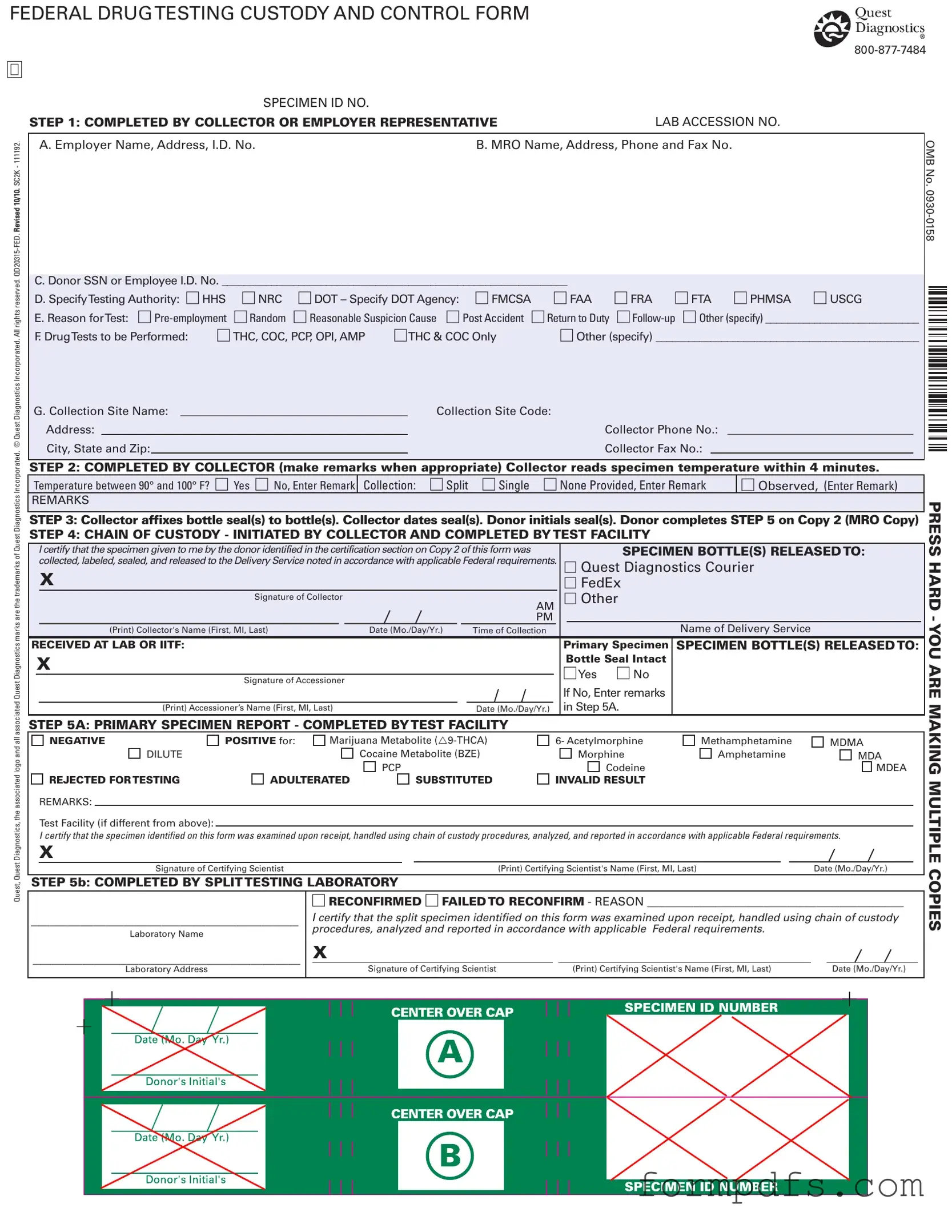
Blank Drug Screen PDF Form
The Drug Screen form is a crucial document used in the drug testing process, especially within various employment and regulatory contexts. This form, officially known as the Federal Drug Testing Custody and Control Form, is designed to ensure that all steps in the drug testing process are properly documented and adhered to. It includes essential sections such as the identification of the employer, the Medical Review Officer (MRO), and the donor's social security or employee ID number. Specific testing authorities can be indicated, including options like HHS and DOT, along with the reason for testing, which may range from pre-employment to post-accident scenarios. The form also details the types of drug tests to be performed, such as THC or cocaine, and requires information about the collection site and the collector's contact details. Following the collection of the specimen, the collector must record temperature readings and ensure that the specimen is sealed and labeled correctly, maintaining a strict chain of custody. This meticulous process is vital for the integrity of the testing results, which can ultimately influence employment decisions and compliance with federal regulations.
More PDF Templates
Futa Form - Maintaining accurate payroll records assists in a smooth form 940 process.
Kde Decklist - Include the quantity for each card to ensure accuracy.
In order to facilitate a smooth transaction, both the seller and the buyer should familiarize themselves with the appropriate documentation, including the importance of using the California Vehicle Purchase Agreement. This form is an essential part of the vehicle sale process that helps clarify the responsibilities of each party. For those seeking additional resources, you can check out All California Forms to ensure you have the correct paperwork needed for your vehicle purchase.
Salary Advance Format - Complete this form if you need an immediate advance against your salary.
Documents used along the form
The Drug Screen form is a critical document in the drug testing process, ensuring compliance and proper handling of specimens. Alongside this form, several other documents are commonly utilized to streamline the testing and reporting process. Below are five important forms that complement the Drug Screen form.
- Chain of Custody Form: This document tracks the handling of the specimen from collection to testing. It ensures that the sample remains uncontaminated and that all individuals involved in the process are documented, providing a clear timeline of custody.
- Medical Review Officer (MRO) Report: After testing, the MRO reviews results and communicates findings. This report may include explanations for positive results and is essential for determining whether a result is legitimate or if it could be due to prescribed medications.
- Consent Form: Before testing, donors must provide consent for their specimen to be collected and analyzed. This form outlines the purpose of the testing and ensures that donors are aware of their rights and the process involved.
- Arizona Bill of Sale Form: To finalize ownership transfers, consider our detailed Arizona bill of sale form requirements for accurate documentation.
- Testing Authority Notification: This document informs the relevant testing authority (such as DOT or HHS) about the testing procedure and results. It helps maintain regulatory compliance and ensures that the testing meets required standards.
- Result Notification Form: Once the testing is complete, this form communicates the results to the employer or designated recipient. It includes details about the findings and any next steps that may be necessary based on the results.
These documents work together to create a comprehensive framework for drug testing. By ensuring that each step is documented and communicated effectively, organizations can maintain integrity and compliance throughout the process.
Form Breakdown
| Fact Name | Details |
|---|---|
| Form Title | Federal Drug Testing Custody and Control Form |
| Purpose | This form is used to document the collection and testing of urine specimens for drug testing in compliance with federal regulations. |
| Governing Law | The drug testing process is governed by the Department of Transportation (DOT) regulations and the Health and Human Services (HHS) guidelines. |
| Testing Authorities | Testing can be conducted under various authorities, including HHS, NRC, and DOT, with specific agencies such as FMCSA, FAA, and others. |
| Collection Process | The collector must ensure the specimen temperature is within the acceptable range of 90° to 100° F and document the collection process accurately. |
| Chain of Custody | The chain of custody must be maintained from the point of collection to the testing facility, ensuring the integrity of the specimen throughout the process. |
More About Drug Screen
What is the purpose of the Drug Screen form?
The Drug Screen form is used to document the collection and testing of urine samples for drug testing purposes. It ensures that the process follows federal guidelines and maintains a chain of custody from the moment the specimen is collected until it is analyzed in the laboratory. This form is crucial for employers and testing facilities to verify the integrity and accuracy of the drug testing process.
Who is responsible for completing the Drug Screen form?
The form is primarily completed by the collector or an employer representative. They are responsible for filling out the necessary information, including the employer's details, the reason for testing, and the specific drugs to be tested. This ensures that all relevant information is accurately recorded and that the testing process is compliant with federal regulations.
What information is required on the Drug Screen form?
Several key pieces of information are required on the form. This includes the employer's name and address, the Medical Review Officer's (MRO) contact information, the donor's Social Security Number or Employee ID, the testing authority, the reason for the test, and the specific drugs to be tested. Additionally, details about the collection site and the collector's information must be provided to maintain transparency and accountability.
What happens if the specimen temperature is outside the acceptable range?
If the specimen temperature is not between 90°F and 100°F, the collector must make a remark on the form. This is important because it may indicate that the specimen has been tampered with or is not a valid sample. The collector is responsible for ensuring the integrity of the sample and documenting any irregularities that may affect the testing results.
What does the chain of custody mean in the context of drug testing?
The chain of custody refers to the process of maintaining and documenting the handling of a specimen from the time it is collected until the testing is completed. This includes recording who collected the sample, how it was sealed, and who transported it to the laboratory. A proper chain of custody ensures that the specimen remains uncontaminated and that the results are reliable and defensible in case of disputes.
What should I do if I receive a positive test result?
If you receive a positive test result, it is important to understand the next steps. You may have the right to request a retest or a split specimen analysis, depending on the policies of your employer and the testing facility. It is advisable to consult with your employer or the MRO for guidance on how to proceed and to discuss any potential implications for your employment.
How is the confidentiality of test results maintained?
Confidentiality is a critical aspect of drug testing. Test results are typically shared only with authorized personnel, such as the employer and the MRO. The Drug Screen form includes provisions to protect the privacy of the donor, ensuring that sensitive information is handled appropriately. Employers must comply with federal regulations regarding the confidentiality of drug test results.
What should I do if I believe my test was conducted improperly?
If you believe that your drug test was conducted improperly, it is essential to document your concerns and discuss them with your employer or the MRO. You may have the right to appeal the results or request a re-evaluation of the testing process. It is crucial to address these issues promptly to ensure that your rights are protected.
How can I contact Quest Diagnostics for questions about the Drug Screen form?
If you have questions regarding the Drug Screen form or the testing process, you can contact Quest Diagnostics at the phone number provided on the form: 800-877-7484. They can assist you with any inquiries related to the testing procedures, results, or other concerns you may have.
Drug Screen: Usage Steps
Completing the Drug Screen form is essential for ensuring compliance with federal requirements regarding drug testing. The following steps outline the process for filling out the form accurately.
- Begin with the section labeled "COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE." Enter the Employer Name, Address, and I.D. No..
- Provide the MRO Name, Address, Phone, and Fax No..
- Fill in the Donor SSN or Employee I.D. No..
- Specify the Testing Authority by checking the appropriate box (HHS, NRC, DOT) and, if applicable, indicate the specific DOT Agency.
- State the Reason for Test by selecting one from the options provided or specifying another reason.
- List the Drug Tests to be Performed by checking the relevant boxes or specifying additional tests.
- Complete the Collection Site Name, Collection Site Code, Address, Collector Phone No., City, State, and Zip, and Collector Fax No..
- In the next section, the Collector must read the specimen temperature within 4 minutes. Indicate whether the temperature is between 90° and 100° F by checking "Yes" or "No" and entering remarks if necessary.
- Specify the type of Collection (Split, Single, None Provided) and enter any necessary remarks.
- The Collector should affix bottle seal(s) to the specimen bottle(s), date the seal(s), and have the donor initial the seal(s).
- Have the donor complete STEP 5 on Copy 2 (MRO Copy).
- Initiate the CHAIN OF CUSTODY by certifying that the specimen was collected, labeled, sealed, and released according to federal requirements. Include the name of the Delivery Service and the Collector's signature.
- Record the date and time of collection.
- In the PRIMARY SPECIMEN REPORT section, indicate whether the specimen is NEGATIVE or POSITIVE for specific substances, and include remarks if necessary.
- Complete the section for the Certifying Scientist, including their signature and date.
- If applicable, complete the section for the SPLIT TESTING LABORATORY and provide the required information and signatures.
After filling out the form, ensure all sections are complete and accurate. The form must be submitted according to the established protocols to facilitate the drug testing process.